Properties of sulfur
| Atomic Number: | 16 |
| Atomic Symbol: | S |
| Atomic Weight (amu): | 32.064 |
| Electronegativity: | 2.58 |
| Melting point: | 112.8°C | 235°F | 385.9K |
| Boiling point: | 444.6°C | 832.3°F | 717.7K |
What does sulfur look like?
Pure sulfur is a light-yellow solid with a brittle texture. It can be found in crystal form and in powdered form. It is not a good conductor of electricity and it is not soluble in water.
What is the biological role of sulfur?
Sulfur is an essential element to all living organisms. It is used to form two of the essential amino acids, cysteine and methionine, which are then in turn used to produce proteins. Certain co-enzymes also require sulfur. The average human consumes approximately 1g of sulfur, mainly through proteins.
What is pure sulfur used for?
- sulfur is used in gunpowder.
- Fungicides are made from sulfur.
- sulfur is also heated with rubber to produce cross-links between the rubber molecules in order to improve the quality of the rubber being produced.
- Sulfur is used to produce sulfuric acid which is used to produce phosphates. Phosphates are the main compound in fertilisers.
What are the main compounds with sulfur
Hydrogen sulfide - it is used in fertilisers to aid as a substitution for salt, it creates other chemicals, and it is the most common form of potassium.
Dimethyl sulfide - is an alkylating agent which prevents the transcription of RNA to DNA and thus inhibits protein synthesis. It also serves as an immunosuppressive agent.
Dimethyl disulfide - has an unpleasant garlic / decaying fish odour. It can be found naturally in many foods and it can also be used as an artificial flavoring agent.
Methanethiol - is used to produce one of the essential amino acids methionine. This essential acid is then used to produce animal and poultry feed.
Carbon disulfide - is a transparent liquid. It is used to produce rubber, cellophane, viscose rayon and carbon tetrachloride.
Carbonyl sulfide - is not produced in large amounts but it is used as an intermediate to manufacture herbicides, which control unwanted vegetation.
Where can sulfur be found?
Sulfur occurs naturally in volcanic areas. It is also found in a variety of minerals such as gypsum, iron pyrates, galena and Epsom salts. All living things contain sulfur and sulfur remains present even after the fossilisation process. Unpurified fossil fuels can release sulfur dioxide into the atmosphere when burnt. This leads to the formation of acid rain.
Is sulfur expensive?
You can buy a bag of sulfur on Amazon:

Two pounds Pure Sulfur fertiliser (Click to view the price on Amazon)
Bag of high quality Sulfur powder crystals. 99.9% pure pastille elemental sulfur for use in organic gardening.
Will we ever run out of sulfur?
Sulfur is a very abundant element and can be found in various sources such as fossil fuels, volcanic deposits, natural gases, meta sulfides and tar sands. The possibility of running out of sulfur is thus not very likely since we have abundant natural sources from which sulfur can be obtained.
Can sulfur be recycled
Sulfur is one of the elements that can be recycled indefinitely. The sulfur cycle provides us with a good idea of how sulfur recycles itself within nature. All living matter containing sulfur releases this sulfur into the ground after a series of transformations through microbes. Amino acids containing sulfur are converted into hydrogen sulfide through another series of microbes found in soil. When exposed to oxygen, the hydrogen sulfide is converted into sulfur which is then converted to sulfate. This last action is possible due to the presence of sulfur bacteria. The sulfur sets into fossils and fossil fuels which are burned to use as a form of energy. The sulfur then enters the atmosphere and eventually enters our water bodies through precipitation. This water enters the soil and the cycle repeats itself.
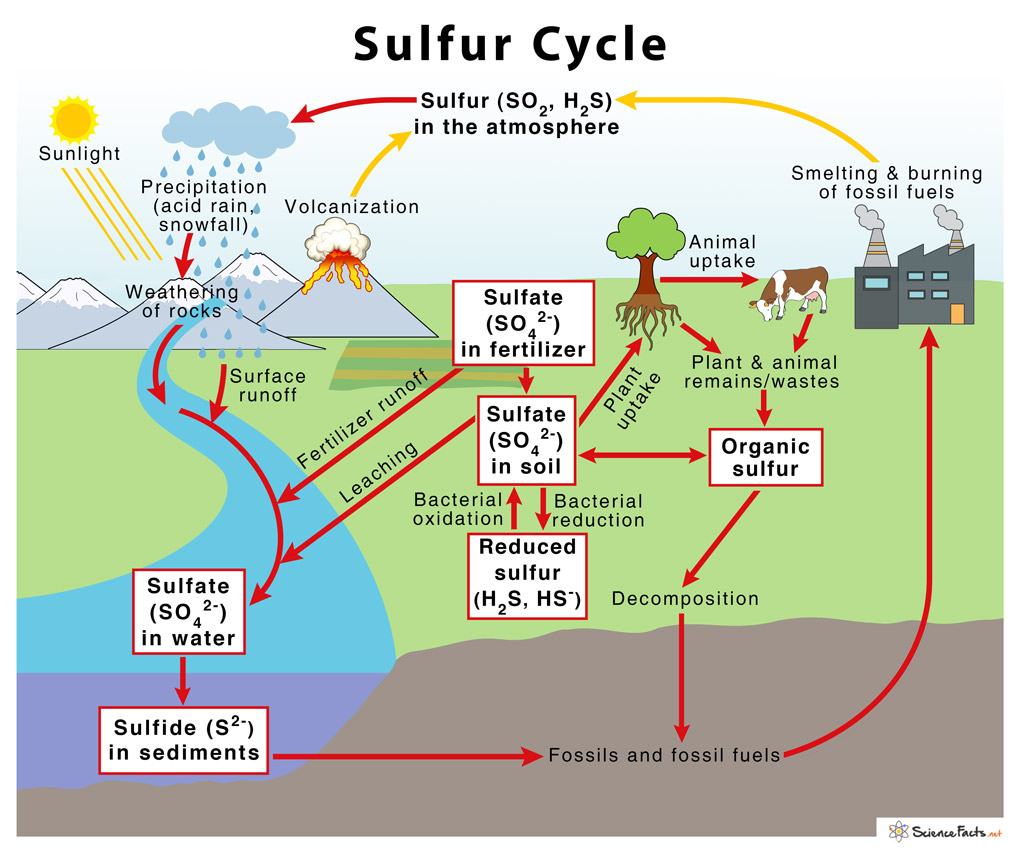
Image by: ScienceFacts.net
Who discovered the sulfur element?
Sulfur is one of the elements that have been known to humans since ancient times. Primitive man used sulfur to create wall art in caves. The ancient name for sulfur was brimstone and was referred to several times in the Bible. In approximately the year 1777, scientist Antoine Lavoisier managed to convince the rest of the scientific community that sulfur is an element. He was able to prove this by burning it down to a red-colored liquid.
Is sulfur dangerous?
Sulfur is not high in toxicity, but if one does consume large amounts of pure sulfur, it can lead to diarrhoea and sensations of burning. If sulfur is inhaled it can also cause irritation to the respiratory airway and can lead to coughing. It can also cause irritation to the eyes and skin. Sulfur is dangerous to aquatic organisms when the concentration thereof is too high. Sulfur is also a combustible and flammable solid and gas and should be handled with care near open flames.
Fun facts about sulfur
- Trace elements of sulfur can be found in the human body and it is important for the growth of hair and the development of nerves. The bad smell that is released from burning hair, is due to the presence of sulfur.
- Hydrogen sulfide gas is present in onions and is the main cause for your eyes tearing up when you cut an onion.
- Most sulfur compounds release a distinctive smell and are present in rotten eggs, and garlic and it is also responsible for the smell of urine after eating asparagus.
- Sulfur was used in an ancient flamethrower weapon which was called Greek Fire.
- Bacteria use sulfur compounds as an energy source.
Funny sulfur Jokes, Puns and One-Liners
Did you hear about all the dating websites made by sulfur companies? They are excellent matchmakers.
We compiled a list of the Top 50 Chemistry Jokes and Puns of all time!



