Is nitrogen the real element of life? And is liquid nitrogen really dangerous?
If you've ever taken a biology class, you'll know about the nitrogen cycle. You'll also know that lightning is an essential component in the process and that without nitrogen our beautiful plant would be much less green than it is today.
Properties of the nitrogen element
| Atomic Number: | 7 |
| Atomic Symbol: | N |
| Atomic Weight (amu): | 14.007 |
| Electronegativity: | 3.04 |
| Melting point: | -210.10°C | -346.1°F | 63.05K |
| Boiling point: | -195.8°C | -320.43°F | 77.36K |
What does nitrogen look like?
Pure nitrogen is an odourless, tasteless, or colorless gas. It is also an inactive element, occurring abundantly in nature both free and as part of industrially significant compounds.
What is pure nitrogen used for?
Liquid nitrogen is formed when nitrogen is cooled to a temperature below -195.8°C. This liquid form of nitrogen is used in endless cooling mechanisms. One of the most common uses is the cryopreservation of cells and microbes.
Similar to helium, nitrogen is also used as an inactive atmosphere, which can be used to preserve food.
Lightbulbs are filled with nitrogen because hot filaments will cause the oxygen in the air to combust
Nitrogen gas can be used as a fire suppressor, while still being at acceptable levels for human consumption.
Nitrogen is also used in the manufacturing of steel as a hardener.
Pure nitrogen is preferred to be used as a tire filler above the air.
Aircraft fuel systems use nitrogen to reduce fire hazards.
Some beers use a combination of nitrogen and carbon dioxide to produce a more smooth taste.
Uses for nitrogen compounds
The applications of nitrogen compounds are extremely widely varied and we will only name a few of the uses of nitrogen compounds:
Nitrogen dioxide is used as an intermediate in the production of nitric acid. Nitric acid is used for the production of ammonium nitrate, a major component of fertilizers.
Ammonia (NH3) is used in agriculture, the manufacture of plastics, explosives, textiles and many more.
Where can nitrogen be found? The nitrogen cycle:
Nitrogen is everywhere and about 78% of the earth’s atmosphere consists of nitrogen.
Nitrogen is an essential element for all life on earth because it forms part of the building blocks of proteins. But how does nitrogen transfer from the air into living species you might wonder? Pure nitrogen in its gas form cannot be absorbed by living organisms and has to undergo a cycle called the nitrogen cycle.
The nitrogen cycle begins with the fixing of nitrogen or nitrogen fixation which is the process where nitrogen gas is combined with oxygen to form nitric oxide. This can occur when the high temperatures of lightning the oxygen and moisture in the are to form nitrates that fall on the ground with rain.
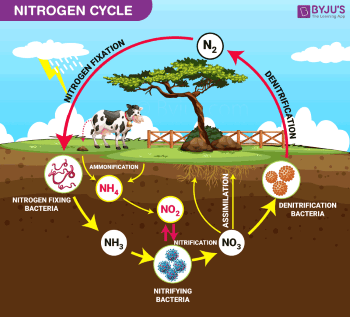
- Pressure swing adsorption
- Membrane nitrogen generation
- Fractional distillation
Is nitrogen expensive?
Pure nitrogen gas is traded at $0.62 per cubic meter.
Who discovered the nitrogen element?
Scientists Carl Scheele, Henry Cavendish, Joseph Priestley, and others concurrently and independently were very close to discovering nitrogen. These scientists managed to isolate nitrogen but did not recognise that it was an undiscovered element.
In 1772 the Scottish scientist Daniel Rutherford published the first article about nitrogen. The name nitrogen was not coined until 1790, by the French chemist Jean Antoine Claude Chaptal, who originally named it “Nitrogène”.
Is nitrogen dangerous
Similar to helium, nitrogen is an asphyxiant, which means that the gas displaces oxygen. One of the properties of nitrogen that makes it dangerous is the fact that it is odourless, which means that in a room where the nitrogen concentration is slowly rising, a human might not realise the rise in nitrogen gas and could fall asleep or pass out and suffocate while being unconscious.
Liquid nitrogen can cause frostbite and cold burns if not handled with extreme care.
Interesting facts about nitrogen
- Guinness beer is carbonated with a mixture of nitrogen and oxygen which give Guinness its smooth taste.
- Nitrogen gas makes up 78.1% of the earth’s atmosphere
- Nitrogen is a component of all proteins and is therefore found in all living organisms. (Yes ALL plants have protein, google it!)
Funny nitrogen Jokes, Puns and One-Liners
What happens to nitrogen in direct sunlight? It becomes daytrogen
What did the Chemist have with his eggs? Barium (Ba) Cobalt (Co) and Nitrogen (N).
We compiled a list of the Top 50 Chemistry Jokes and Puns of all time!



